The surge in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections has warranted widespread, frequent, and continued testing to identify and mitigate the outbreaks of novel epidemics. Although nasal middle-turbinate and nasopharyngeal swabs are routinely obtained for standard COVID-19 testing, they are associated with increased patient discomfort and require manual collection.
The persistent nature of the pandemic could lead to psychological fatigue, decreasing public sensitivity toward COVID-19 control. In this regard, passive surveillance systems such as fully automated smart toilets that enable the detection of SARS-CoV-2 ribonucleic acid (RNA) in fecal matter could be a valuable tool in COVID-19 diagnostics.
Notably, previous studies have reported that SARS-CoV-2 can be detected in feces even after the resolution of pulmonary symptoms, and approximately five weeks after the SARS-CoV-2 RNA detection tests yield negative results.
Of interest, a previous publication of the authors of the present study showed that smart home occupants are more accepting of smart toilets. Thus, the team envisioned passive smart toilet-based testing as a key strategy in advancing precision health-based COVID-19 diagnostics.
 Study: Smart toilets for monitoring COVID-19 surges: passive diagnostics and public health. Image Credit: Pixel-Shot / Shutterstock
Study: Smart toilets for monitoring COVID-19 surges: passive diagnostics and public health. Image Credit: Pixel-Shot / Shutterstock
About the study
In the present study, researchers reported the potential use of smart toilets for passive and automated COVID-19 diagnosis and longitudinal surveillance.
The COVI-ID smart toilet platform comprises bidet-style mountable attachments for easy installation in highly populated public spaces such as shopping complexes, sports arenas, hospitals, and schools. They do not need the replacement of the existing infrastructure. The smart toilets have modules for fully automized and fecal specimen collection and ultrafast SARS-CoV-2 RNA detection using in situ nucleic acid amplification tests (NAAT). In addition, smart toilet technology ensures efficient sanitization.
Smart toilets have in-built Quick Response (QR) codes which can be scanned when an individual sits down to defecate, and stool samples can be automatically obtained. Within minutes, the test findings are obtained and uploaded to a centralized and anonymized tracing network. These tracing networks could be augmented to yield real-time data for determining COVID-19 prevalence. This would aid in preventing epidemic and local outbreaks by detecting the etiological agents.
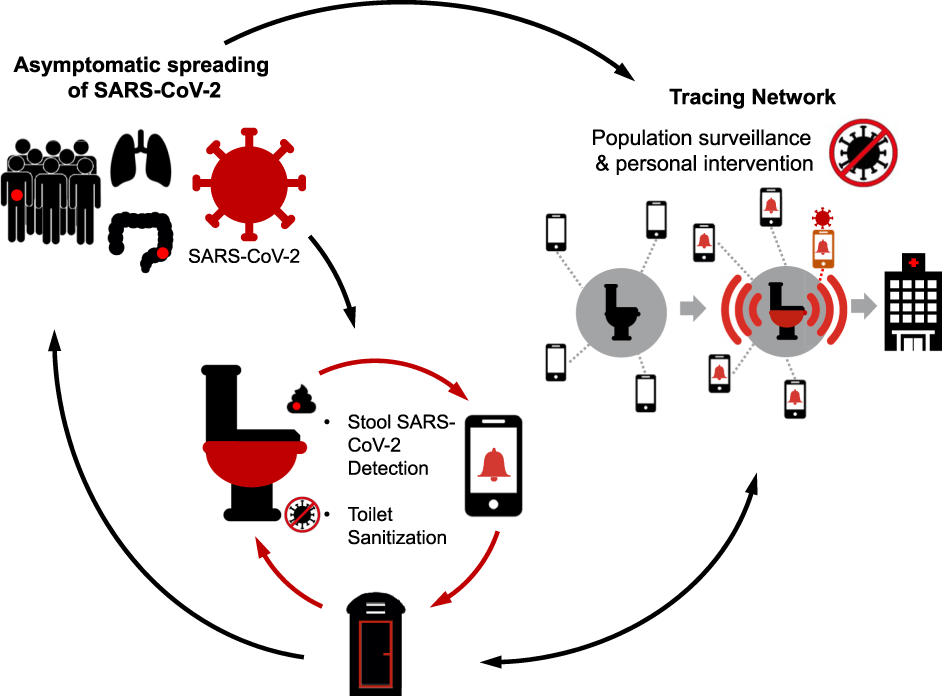
The COV-ID toilet will serve as a centralized diagnostic center in essential businesses to detect COVID-19 infection amongst the asymptomatic populations and to facilitate early diagnosis and isolation. Stool sampling will be automated with a quick turn-around time for viral RNA testing, and the toilet seat/surfaces will be sanitized in between users. The user will register with the health portal or a tracing network (which can be performed on a mobile device while on the toilet), which will then alert them to their test results (ideally within 15 min), link them with medical care, provide advice and guidelines from physicians for isolation. Aggregate data can also be used for population surveillance to assess community burden.
Furthermore, the tracing system of the COV-ID platform could be linked via Bluetooth to Google and Apple devices and uploaded to individual smartphones, if individuals consented to provide their mobile numbers. Individuals with positive test results would also be provided information on the required quarantine protocols and confirmatory tests.
Certain prerequisites have to be met for the use of smart toilets for COVID-19 surveillance. First, the turnaround time must be minimal (ideally 15 minutes). This is completed by using ultrafast speed NAAT for viral RNA detection, which shortens detection time from several hours in standard NAAT methods such as polymerase chain reaction (PCR) to less than 15 minutes. Additionally, parallel runs of SARS-CoV-2 detection in the COV-ID system ensure rapid toilet availability for users.
Manual collection of stool samples is an uncomfortable and odious procedure that would limit the use of fecal matter for COVID-19 detection. Thus, the sample collection procedure of the COV-ID platform was fully automated. This decreased human intervention and increased patient compliance.
Furthermore, the toilets must be hygienic and prevent cross-contamination among the COVID-19 test samples. This prerequisite could be met by using ultraviolet sterilizers for rigorous sanitization of toilet surfaces and analytical devices in the COV-ID platform.
Smart toilets being an Internet of Things (IoT) technology-based system must be able to securely connect to centralized tracing networks to enable active user participation and aid determination of COVID-19 epidemiology that would aid in formulating COVID-19 guidelines and prevent outbreaks in the future. This was met by using centralized tracing and transferring information via Bluetooth to Google and Apple smartphone devices in the COVI-ID platform.
Another important prerequisite for COV-ID success was the maintenance of user privacy. The smart toilet platform would de-identify individual user information and only upload them to the centralized tracing network if the user elected to provide their cell phone numbers.
In addition to COVID-19 diagnosis, the COV-ID platform could provide supplemental information on the oxygen saturation levels and body temperature, which are important parameters to assess COVID-19 severity. The COV-ID platform also enabled the analysis of stool morphology, which could detect diarrhea, a potential COVID-19 symptom.
To conclude, according to the study findings, the COV-ID smart toilet platform provides a non-invasive, rapid, and convenient approach for passive and longitudinal monitoring of COVID-19. This platform enables early COVID-19 detection in asymptomatic infected individuals and thereby facilitates the prevention of potential outbreaks. However, the platform’s success largely depends on user consent and acceptance.